It can be overwhelming to stay up-to-date on information about COVID-19, and that includes information about diagnostic testing. At the start of the crisis, coronavirus testing in the U.S. was severely limited, but that is changing. Now there are not one, not two, but three distinct types of COVID-19 tests, and IGeneX is proud to offer some of them.
However, there are significant differences in the methodologies, purposes, and availability of these coronavirus tests. This article will clear up any confusion you may have about what types of diagnostic tests are currently available for COVID-19.
Guide to COVID-19 Tests
The current COVID-19 tests can be broken down into two general categories: tests that detect current infections vs. tests that detect past infections. Of the tests that detect current infections, the most common is the Reverse Transcription Polymerase Chain Reaction (RT-PCR) test, but antigen tests are also available. We will cover both of these types of diagnostic tests below before moving on to antibody testing.
Diagnosing Current Infections
 1. RT-PCR Tests
1. RT-PCR Tests
PCR tests – also known as molecular tests or nucleic acid amplification tests (NAAT) – are the most widely used type of diagnostic test for COVID-19. Though the terms PCR and RT-PCR are sometimes used interchangeably, most currently available PCR tests are in fact RT-PCR tests. Both traditional PCR tests and RT-PCR tests are reliable, but they use different methods for expanding DNA chains from viral samples. RT-PCR tests work by detecting genetic material (RNA) from the virus itself in specimens collected from the nose, throat, or more recently saliva.
Many experts agree that RT-PCR tests are currently the most reliable method for detecting active infections. However, false negatives are possible and can be caused by several factors:
- There may not be enough viral material in the sample to cause amplification.
- RT-PCR tests require many resources as well as expert interpretation in a lab. Defects in testing materials (such as swabs) or human error can result in inaccurate test results.
- The magnitude of the crisis has required tests to be developed very quickly, meaning there may be more variability than usual in the quality and accuracy of available tests.
Because of these limitations, it is best to consider RT-PCR test results in the context of a patient’s symptoms and exposure history.
2. Antigen Tests
Antigen tests are sometimes referred to as “rapid tests.” Like RT-PCR tests, antigen tests require specimens from the nose, throat, or saliva. However, instead of detecting genetic material from the virus, antigen tests detect proteins produced by the virus. They are similar to rapid strep tests or flu tests already used in many doctor’s offices.
While antigen test results can be obtained in minutes, they are not as accurate as RT-PCR tests. Antigen tests typically have lower sensitivity, meaning that a positive result is highly accurate, but a negative result may not mean that you aren’t infected.
Because antigen tests are more prone to false negatives, they are recommended to be used as quick screening tests to be confirmed with something more sensitive, like a RT-PCR test.
Detecting Past Infections
Antibody Tests
Antibody tests came on the scene later than diagnostic tests. You may have heard them referred to as “serology” because they use blood samples rather than nose, throat, or saliva samples. These tests are a form of indirect testing, used to detect not the virus itself, but antibodies produced by the immune system in response to exposure to the virus. This is the same technology used in the current Lyme disease tests.
Because antibody tests only detect exposure to the virus, it is not recommended that they be used to diagnose active infections. It can take days or weeks for the body to develop enough antibodies to show up on a serological test. There are two antibody responses. The first response is the IgM antibody followed by IgG. Most tests on the market are only testing for IgG antibodies and so positive results on most antibody tests thus typically mean that the patient is in recovery or has already recovered. However, when a patient has IgM antibodies, it indicates a recent infection and they may not have fully recovered. As mentioned above, sometimes PCR tests have a false negative result. An IgM antibody test would be useful in that situation.
So if not for diagnostic purposes, what is an antibody test used for? Widespread antibody testing can provide valuable information about what proportion of the public has actually been exposed to the virus, which can help researchers further understand whether past exposure provides immunity to new infection and for how long.
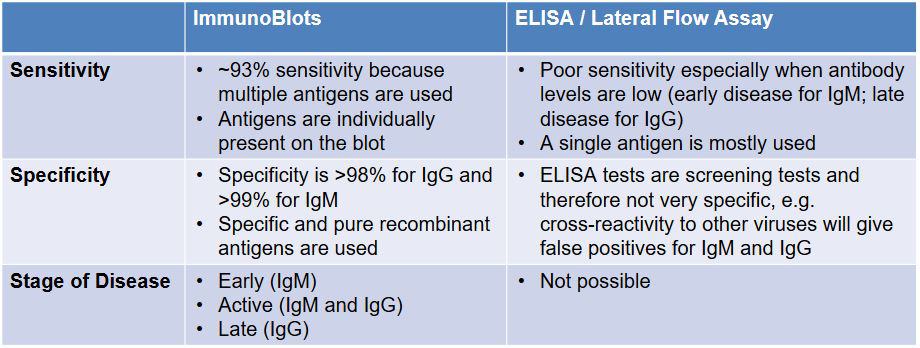
ImmunoBlot vs. Lateral Flow Assay and ELISA
There are currently several types of antibody tests on the market. Like the types of diagnostic tests discussed above, there is variability in the accuracy and quality of these tests. This stems partly from the actual methodology of the tests.
For example, two types of commonly available current tests are Lateral Flow assays and ELISA tests. Lateral Flow assays are rapid, but lack sensitivity and specificity. ELISA tests are notorious in the Lyme community for resulting in high numbers of false negatives, and currently there is a lack of data on whether ELISA tests for COVID-19 are as accurate as they claim to be. The IGeneX ImmunoBlot for IgM and IgG, on the other hand, uses recombinant proteins – as in the Lyme ImmunoBlot – to provide higher sensitivity and specificity.
Which test should I take?
Knowing which is the best test to take for your situation is important, since – as mentioned above – the tests that detect current infections are different from the tests that detect past infections.
- Early Infection – At and right after the onset of symptoms, the virus is detectable by RT-PCR, as shown in the blue line below. RT-PCR tests can detect the virus for up to three weeks after the onset of symptoms.
- Week 2 – By week 2, IgM antibodies may be detectable by antibody test, as shown by the gray dotted line below. IgM antibodies may remain detectable for up to 1 month after the onset of symptoms.
- Week 3 – By week 3, the body may have begun producing IgG antibodies, as shown in the orange dotted line below.
- Late Infection – Between weeks 3 and 6, some patients have both IgM and IgG antibodies, as shown in the blue dotted line below.
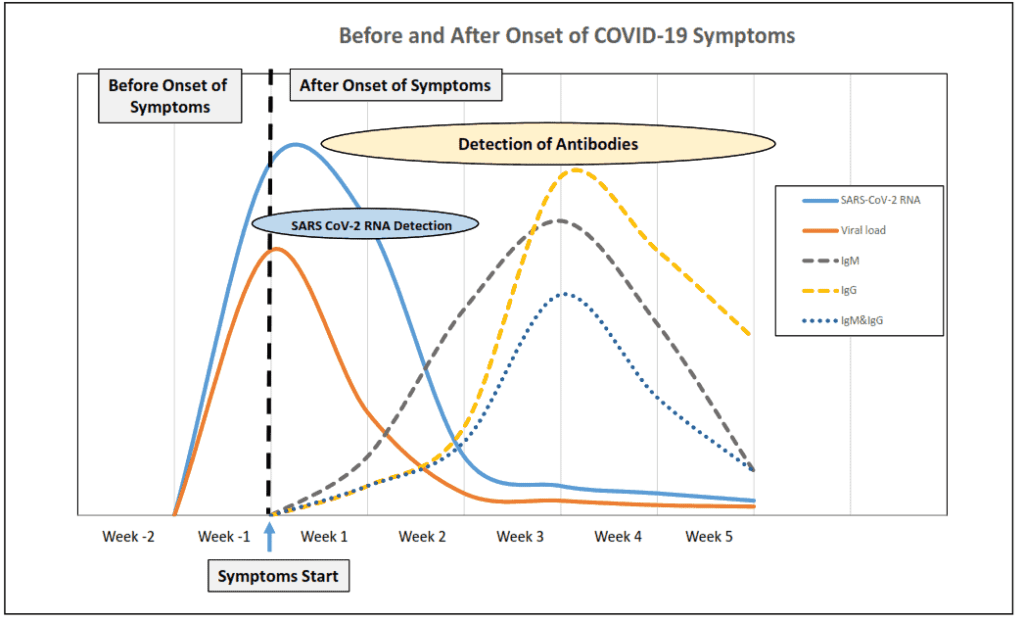
A person is considered to have active disease if he or she receives a positive RT-PCR or if IgM antibodies are detected. Also, a patient might have a second infection, in which case the patient would be positive for IgG antibodies and by real-time RT-PCR.
Testing Regulations
The FDA implements Emergency Use Authorization (EUA) to ensure that tests are developed and delivered quickly yet safely. The EUA allows entities to bypass the arduous FDA-approval process in order to respond to the crisis rapidly. EUA can be granted to unapproved medical tests or equipment, or for the unapproved use of approved medical tests or equipment, in the event of an emergency such as the COVID-19 pandemic.
A list of currently authorized coronavirus tests can be found on the FDA website. Note that as of right now, there are no authorized coronavirus tests that are able to be taken and read completely at home.
CDC Recommendations
Though testing has become much more widely available in recent weeks, the CDC still recommends prioritizing certain high-risk, symptomatic patients first. Observe the following summary of their recommendations:
- Those deemed high priority include hospitalized patients with symptoms; healthcare facility workers, workers in congregate living settings, and first responders with symptoms; and residents in long-term care facilities or other congregate living settings, including prisons and shelters, with
- Those deemed priority include people with symptoms of potential COVID-19 infection (fever, cough, shortness of breath, chills, muscle pain, new loss of taste or smell, vomiting or diarrhea, and/or sore throat); and people without symptoms who are prioritized by health departments or clinicians, for any reason, including but not limited to: public health monitoring, sentinel surveillance, or screening of other asymptomatic individuals according to state and local plans.
Decisions about testing are ultimately made by state and local healthcare departments and providers. The CDC recommends that you contact your doctor first if you think you need to be tested for COVID-19.
Looking Ahead
As researchers and medical professionals gather more information about COVID-19, the hope is that testing accuracy and availability will continue to improve. IGeneX is proud to be a part of this effort by offering both real-time RT-PCR and antibody tests with high specificity and sensitivity. Learn more about the IGeneX coronavirus tests today.

