We look for more and detect more, no matter when you were exposed
WHY IGENEX IS DIFFERENT
Dedicated R&D
We have a dedicated research and development team of tick-borne disease experts who are relentlessly focused and committed to developing the most precise and effective diagnostic tests for Lyme disease, Tick-Borne Relapsing Fever, and other tick-borne diseases.
– First to identify Borrelia burgdorferi in ticks in California
– First to identify Babesia in New York, Switzerland, and Australia, using our exclusive patented FISH technology
More Comprehensive Testing
We test for more relevant strains of tick-borne pathogens than any other lab, even those pathogens that exhibit Lyme-like symptoms but may be due to a different tick-borne disease and require different treatment paths.
It is important to test for co-infections. Nearly 1 in 4 ticks infected with the Lyme Disease pathogen carry more than one pathogen. That means nearly 25% of patients will not be diagnosed if the patient is tested only for Lyme disease.
Superior Testing Criteria
We developed our in-house testing criteria for Lyme disease and other tick-borne diseases based on decades of evidence and validation studies. Most labs use Lyme disease testing criteria developed in 1994 when much less was known about the disease and its pathogens.
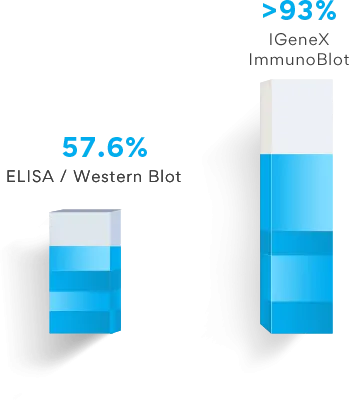
IGeneX has developed several industry-leading tests. Our ImmunoBlot test has a sensitivity rate that is nearly double that of the standard two-tier testing protocol.
Cutting Edge Technologies
We arm our talented scientists with the most cutting-edge technology available to help enable them to find new solutions that challenge the status quo of testing for Lyme and associated tick-borne diseases. This positions us at the forefront of providing the most comprehensive testing possible to aid physicians in diagnosis.
– First to introduce Relapsing Fever Western Blot testing
– First to introduce comprehensive Lyme ImmunoBlot and TBRF ImmunoBlot testing
We are part of the Lyme Community
Lyme disease is the most well-known tick-borne illness and one of the fastest-growing infectious diseases in the United States. It has now been detected in all 50 US States.
IGeneX is deeply dedicated to physician education around Lyme. We are committed partners with Lyme experts and Lyme groups, including ILADS, Bay Area Lyme Foundation, and the Liv Lyme Foundation. IGeneX is also fully CLIA/CMS certified.
Better Detection
IGeneX tests are based on the latest findings of Lyme disease and other tick-borne illnesses to provide the most complete and accurate results for diagnostic purposes. We test for more species than any other lab.
IGeneX offers a broad range of individual tests and panels for all tick-borne diseases. Physicians are encouraged to test their patients using a panel approach, which is a combination of direct and indirect test methodologies that detect multiple tick-borne diseases.
IGeneX ImmunoBlots Are Superior Lyme Disease Tests
Lyme disease has become increasingly challenging to diagnose, making accurate and reliable testing even more essential to inform and support physician diagnoses. The two-tier ELISA/Western Blot testing recommended by the CDC uses result criteria developed in 1994 when far less was known about Lyme disease. These tests don’t provide physicians with a complete picture for accurate diagnosis.
IGeneX ImmunoBlots use recombinant proteins to detect Lyme IgM and IgG antibodies to more B. burgdorferi sensu lato species than the current Western Blot does. Prepared from two strains of B. burgdorferi, the IGeneX ImmunoBlot produces a higher sensitivity and the most comprehensive testing for Lyme disease antibodies in patient serum samples.
Lyme ImmunoBlot IgM
IgM antibodies are the earliest to appear in response to a patient’s exposure to the disease.
IGeneX Specific Proteins (Band-kDA):
23kDa, 31kDa, 34kDa, 39kDa, and 41kDa
IGeneX Result Criteria:
Positive Result:
Combination of any two or more of the above bands
Negative Result:
Any profile that does not meet positive criteria
CDC recommended Specific Proteins (Band-kDA):
23kDa, 39kDa, and 41kDa
CDC Result Criteria:
Positive Result:
Combination of two or more of the above bands
Negative Result:
Any profile that does not meet positive criteria
Lyme ImmunoBlot IgG
Lyme IgG antibodies are produced four-to-six weeks following infection and can persist for years.
IGeneX Specific Proteins (Band-kDA):
23kDa, 31kDa, 34kDa 39kDa, 41kDa, and 93kDa
IGeneX Result Criteria:
Positive Result:
Combination of any two or more of the above bands
Negative Result:
Any profile that does not meet positive criteria
CDC recommended Specific Proteins (Band-kDA):
18kDa, 23kDa, 28kDa, 30kDa. 39kDa, 41kDa, 45kDa, 58kDa, 66kDa, and 93kDa
CDC Result Criteria:
Positive Result:
Combination of five or more of the above bands
Negative Result:
Any profile that does not meet positive criteria

The ImmunoBlot test for Lyme disease has a sensitivity greater than 93%, whereas the ELISA and Western Blot two-tier testing protocol recommended by the CDC has a sensitivity of only 57.6%.
If you suspect, get yourself checked.
IGeneX Tick-Borne tests are the most complete and accurate tests available.










