IGeneX Culture Enhanced PCR Testing
CULTURE TESTING NOW AVAILABLE!
Introducing culture-enhanced PCR testing for the detection of tick-borne disease pathogens
CULTURE TESTING NOW AVAILABLE!
Culture Testing for Tick-Borne Illnesses: An Overview
Culture testing, a technique of taking a sample of a pathogen and allowing it to grow over a period of time, is widely considered to be the “gold standard” for diagnosis of tick-borne illnesses. Until now, culturing tick-borne pathogens has been very difficult and too expensive to be used for clinical diagnosis.
After many years of research and development, IGeneX is pleased to introduce cePCR™ (Culture Enhanced PCR) for all of the major tick-borne illnesses. IGeneX is currently the only lab that can perform culture testing for these diseases.
UNDERSTANDING HOW cePCR Works
In culturing, a clinical sample from the body (e.g. blood) is incubated in media. During this incubation period, micro-organisms in the sample grow and multiply. The sample is then tested by PCR to identify the pathogens.
Step 1
An aliquot of whole blood is placed into a proprietary culture media.
Step 2
The culture media is incubated for several days.
Step 3
PCR tests are performed on the cultured samples.
WATCH WEBINAR ON CULTURE TO LEARN MORE
Who is the webinar for?
This webinar is perfect for those who want to take their tick-borne disease testing to the next level by including culturing in their testing mix.
In this webinar, you will learn:
- The advantages of culture testing for tick-borne diseases
- Why including culturing is essential for a complete picture of your patient
- Why immune deficient patients may have a negative serology but a positive culture
- Why patients with inactive disease (or those on medication) may have a negative culture but a positive serology
- View actual patient data that will help you in your practice!
Download the slides here.
cePCR: VALIDATION STUDIES
IGeneX performed two validation studies to verify the accuracy of the Culture Enhanced PCR Testing method, often referred to as cePCR, to ensure its efficacy.
PCR Culture and Sequencing Validation
IGeneX cultured 1200 samples for two weeks. 57 samples were positive, spread across six diseases listed in the table below. The 57 samples were then sent for DNA sequencing to confirm the species. Some of the species that were detected, such as A. platys, are rarely found in the US, and likely would not be detected with traditional PCR.
1200 Samples Tested (57 Samples Positive)
Lyme Disease
- B. burgdorferi (17)
- B. garinii (2)
- B. mayonii (4)
Tick-Borne Relapsing Fever
- B. miyamotoi (4)
- B. hermsii (1)
Babesia
- B. microti (12)
- B. duncani (7)
- Babesia sp. (1)
Bartonella
- B. henselae (5)
- B. elizabethae (1)
- B. tribocrum (1)
Anaplasma
- A. phagocytophilum (1)
- A. platys (1)
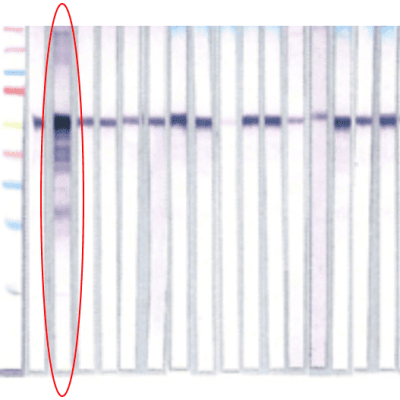
Culture Test and Reverse Western Blotting Validation
IGeneX cultured patients’ blood for 16 weeks and prepared Western blots from the culture pellets. The blots were tested against antibodies to four tick-borne disease groups: Lyme disease, Tick-Borne Relapsing Fever, Babesia, and Bartonella. The blot below clearly shows the detection of a pathogen.
SENSITIVITY AND SPECIFICITY
Sensitivity in cePCR Culture Testing
- IGeneX was able to grow multiple pathogens in culture
- If a pathogen grows in culture, it is guaranteed to be active and not a remnant of a prior pathogen or past infection
Specificity in Culture Enhanced PCR Testing
- All sequencing results matched the initial PCR determination
- All reverse western blots matched exactly the results of sequencing
ADVANTAGES OF cePCR
-
Improved Sensitivity
More sensitive than standard PCR testing. -
Highly Specific
100% specific for identification of a tick-borne disease. -
Comprehensive Screening for Tick-Borne Diseases
Available for Lyme disease, Tick-Borne Relapsing Fever, Bartonellosis, Babesiosis, Ehrlichiosis, Anaplasmosis, and Rickettsiosis
AVAILABLE CULTURE TEST PANELS
IGeneX offers a diverse range of Culture Enhanced PCR Testing panels tailored to specific requirements. Doctors and patients can opt for individual disease testing or select from our comprehensive test panels.
Borreliosis cePCR Test Panel
Includes culture, plus PCR for Lyme and TBRF.
Co-infection cePCR Test Panel
Includes culture, plus PCR for Babesia, Bartonella, HME, HGA, and Rickettsia.
Tick-Borne Disease cePCR Test Panel (Special offer on this panel!)
Includes culture, plus PCR for Lyme, TBRF, Babesia, Bartonella, HME,
HGA, and Rickettsia.
Please note: Medicare does not cover blood culturing. There is a $500 charge that is the responsibility of the patient for any cePCR tests. An ABN is required in advance for all Medicare beneficiaries.

